Water | Part 2.3 - Oxygenation via Nanobubbles
Nanobubble characteristics, generation and application
In our water quality series, we have covered solids, DO and nitrogen in water thus far. This week, let’s dive into oxygenation, specifically nanobubble aeration.
Maintaining suitable levels of dissolved oxygen (DO) is of utmost importance for fish farms to: 1) prevent growth limitation and death of farmed stocks, and 2) ensure the capacity of nitrifying bacteria to breakdown organic waste.
Traditionally, operators aerate aquaculture farms using paddlewheels or airstones. Recently, we’ve witnessed increased integration of nanobubbles on fish farms.
What are nanobubbles?
Nanobubbles or ultrafine bubbles are pockets of suspended gases with diameter <200nm (Chaplin, 2017) and move per Brownian motion within a water column. The smaller diameter reduces buoyancy which allows for longer suspension and homogenous distribution of oxygen throughout the water column. Tekile, Kim and Lee (2016) reported that air and pure oxygen nanobubbles can remain suspended in water for 10 to 15 days respectively until dissolving.
Nanobubbles exhibit high specific surface areas, allowing for increased physical absorptions and chemical reactions at gas-liquid interfaces. Nanobubbles have more than 400 times the surface area of a typical microbubble (measured at 40 micrometers in diameter).
Known as Zeta-potential, the electrically charged liquid-gas interface of nanobubbles create repulsive forces that prevent bubble coalescence and is key to nanobubble stability.
Compared to conventional aeration technologies, which achieves < 3% oxygen transfer efficiency at standard conditions per foot of water, nanobubbles can achieve > 80% of oxygen transfer efficiency, allowing for higher dissolved oxygen levels with far less oxygen and energy usage. The collapse of nanobubbles releases chemically reactive free radicals i.e. unpaired electrons, which oxidize organic compounds and may improve water quality on farms.
More benefits of nanobubbles listed here.
Zeta-potential (ζ-potential)
Defined as the charge that develops at the interface between a solid surface and its liquid medium i.e. surface charge of nanoparticles in solution
Measured in millivolt (mV), Zeta-potential is a scientific term for electrokinetic potential in colloidal dispersions.
How is a surface charge formed?
When a particle suspends in liquid, the functional groups at its surface reacts with the surrounding medium and forms a surface charge. This attracts the accumulation of oppositely charged ions.
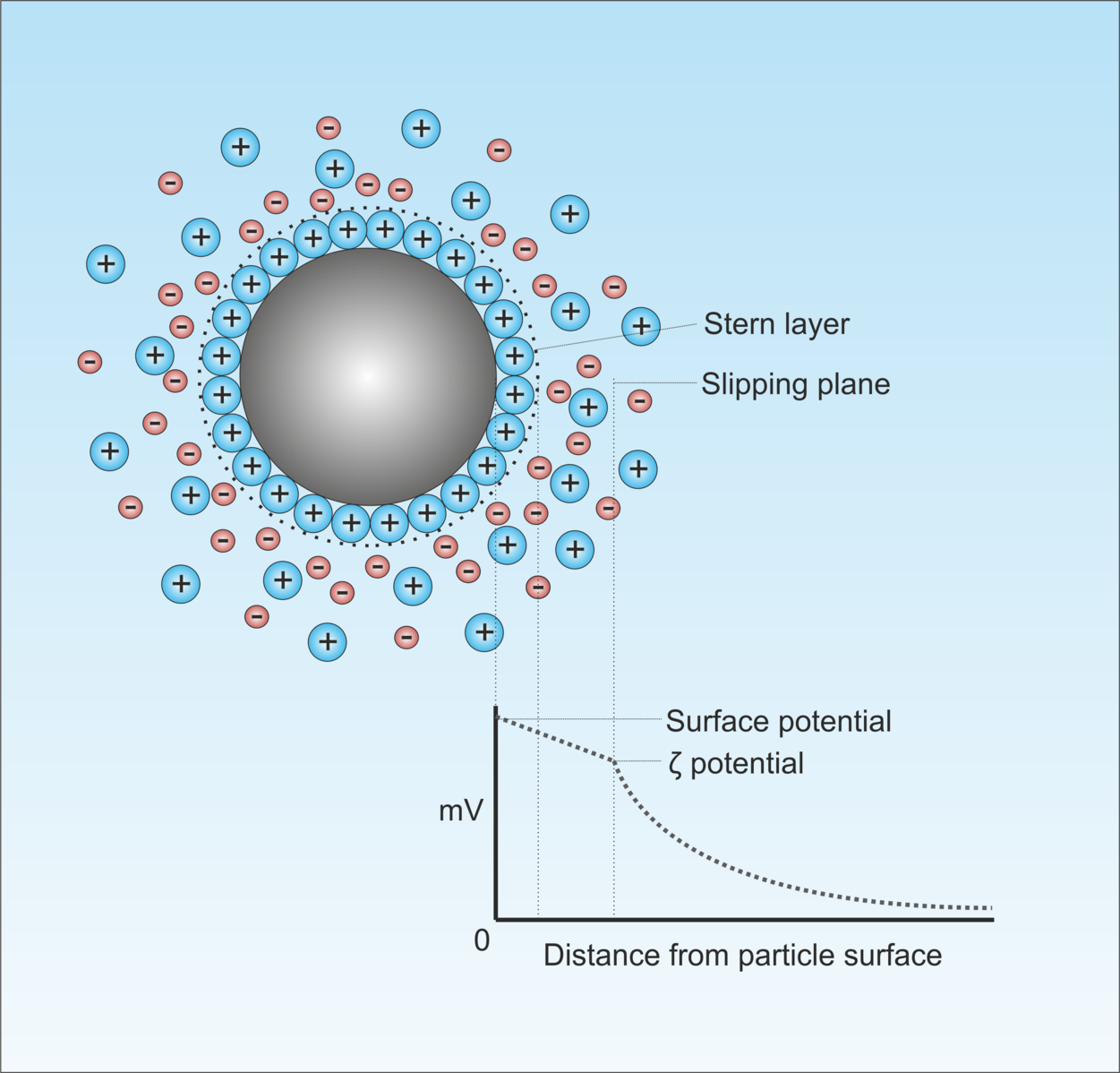
In a colloidal system (defined as a type of mixture in which one part is dispersed constantly throughout another), dispersed particles form two layers of oppositely charged ions at the surface: 1) the layer closer to the particle where ions bound strongly together is known as the stern layer, 2) the layer further away from the particle where ions are loosely bound is known as the diffuse layer. Located at the edge of the diffuse layer, the slipping plane marks the line where the diffuse layer meets the surrounding liquid. Zeta potential measures the voltage at the slipping plane.
If the charge of nanoparticles falls below a certain level, repulsive forces surrounding the particle decreases causing instability. Thus, ζ serves as the basis for understanding the state of nanoparticle surface and predicting the long-term stability of a colloidal dispersion.
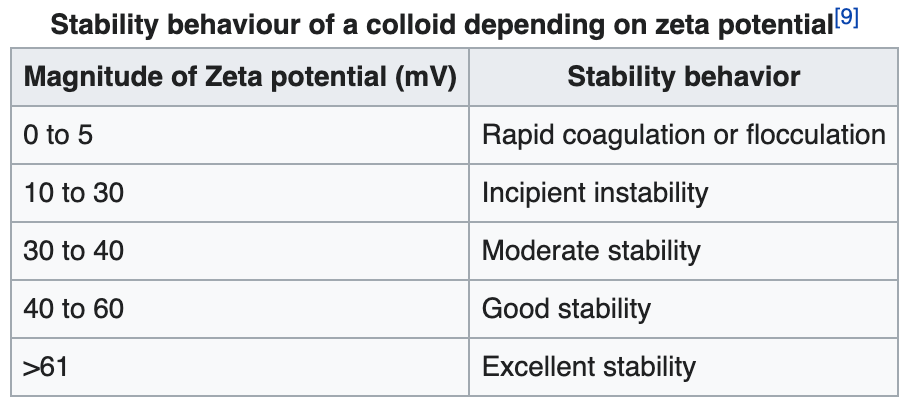
Zeta potential is influenced by infilled gas type, pH, buffer concentration, temperature and ionic strength.
How are nanobubbles generated?
Nanobubbles are created by generating air pockets via pressure reduction methodologies. There are 4 pressure reduction mechanisms i.e. 4 ways of creating bubbles:
hydrodynamic (most cost-effective and efficient) - Hydrodynamic is a branch of physics concerned with forces acting on or exerted by fluids (especially liquids). Simply put, to create a bubble, you need to add gas to a moving liquid, this brings force on the gas and liquid and generates bubbles. For example, when you pop open a can of coke, the change in pressure (force) creates bubbles.
There are 2 common methods to generate nanobubbles hydrodynamically:
Gas-water circulation = compress gas flows in liquids then releasing mixture through nano-sized nozzles to create nanobubbles
Pressurized dissolution (also known as gas-water pressurization-decompression) = inject low pressure gases into liquids and subsequently breaking gas into bubbles via focusing, fluid oscillation, or mechanical vibration
acoustic - apply ultrasound to liquids to create gas pockets acoustically (detailed paper here)
optic - short-pulsed lasers focused into low absorption coefficient solutions (detailed paper here)
particle - pass high intensity light photons in liquids (detailed paper here)
Systems for Nanobubble Generation
Pressurized Dissolution
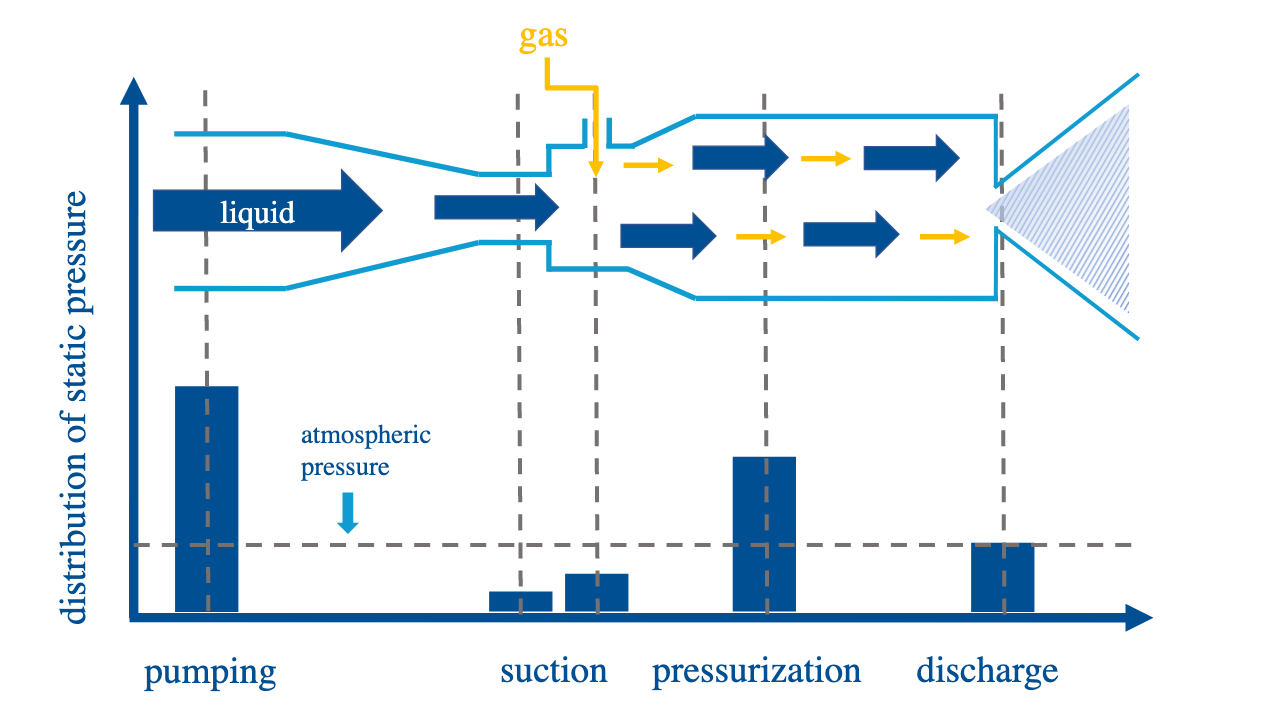
Built upon the principles of Henry’s law, pressurized dissolution creates bubbles by altering liquid pressure.
Henry's law states that the weight of a gas dissolved by a liquid is proportional to the pressure of the gas upon the liquid i.e. the higher the pressure, the more gas a solution can dissolve.
Liquid and gas is mixed together in a venturi system. Liquid is first pumped through a pipe under pressure. The subsequent reduction of the diameter of the pipe increases the speed of liquid thus converting pump pressure into dynamic pressure. This reduces static pressure and allows gas/air to be suctioned into the system via negative pressure. Once the liquid becomes saturated with air/bubbles, the liquid/gas flow flows through a wider pipe and speed of liquid decreases. This converts dynamic pressure back to static pressure and pressurized dissolution of gas takes place. Once gas completely dissolves in liquid, the liquid/gas is ejected at atmospheric pressure resulting in over-saturation and the release of nanobubbles.
Rotational Flow
Known as Swirl Method or Spiral Flow, rotational flow method is based on Bernoulli's principle where an increase in the speed of a fluid occurs simultaneously with a decrease in pressure or a decrease in the fluid's potential energy.
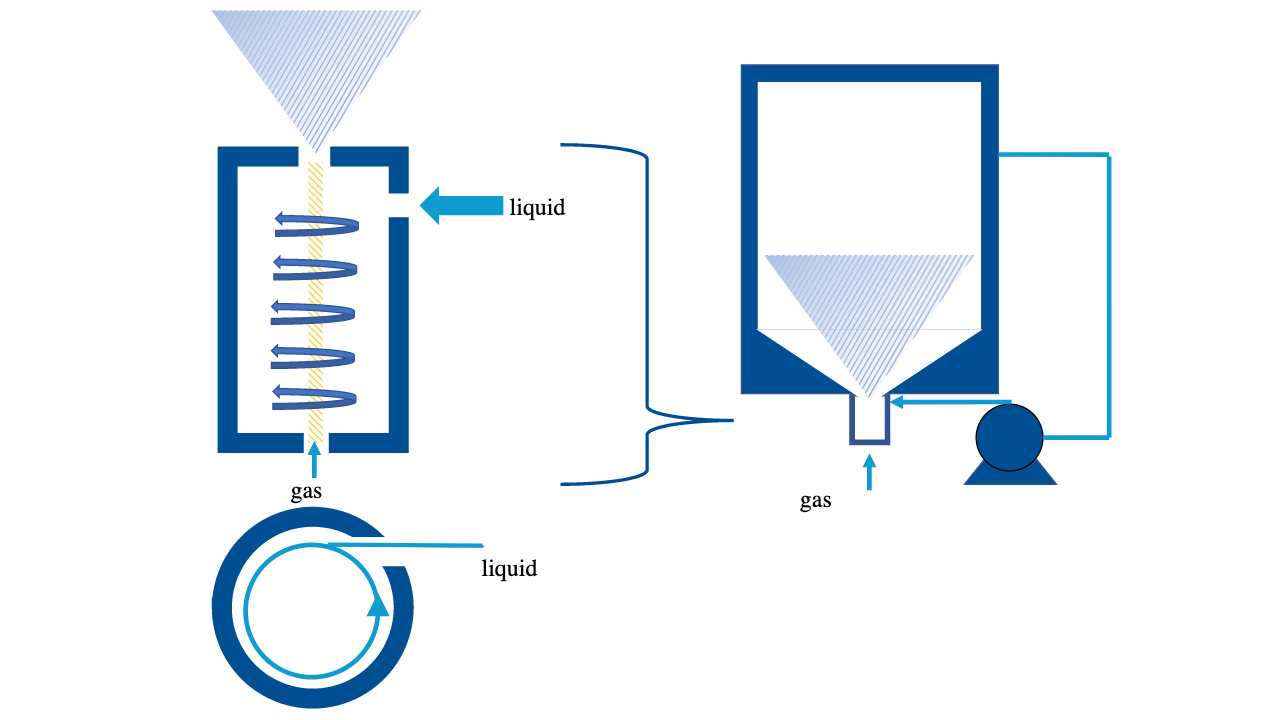
Water spirals downwards in a tank and gas is suctioned in from the bottom of the cylinder tank. Fine bubbles are produced when the rotating water is sheared to the top of the cylinder.
Leveraging Bernoulli’s principles, the first swirl machine produced was the Ranque-Hilsch Vortex Tube in 1933. 50 years later, Swirling jet flame was invented. In the mid-nineties, the 1st swirling type micro-bubbles was invented in Japan.
Turbulent Static Mixer
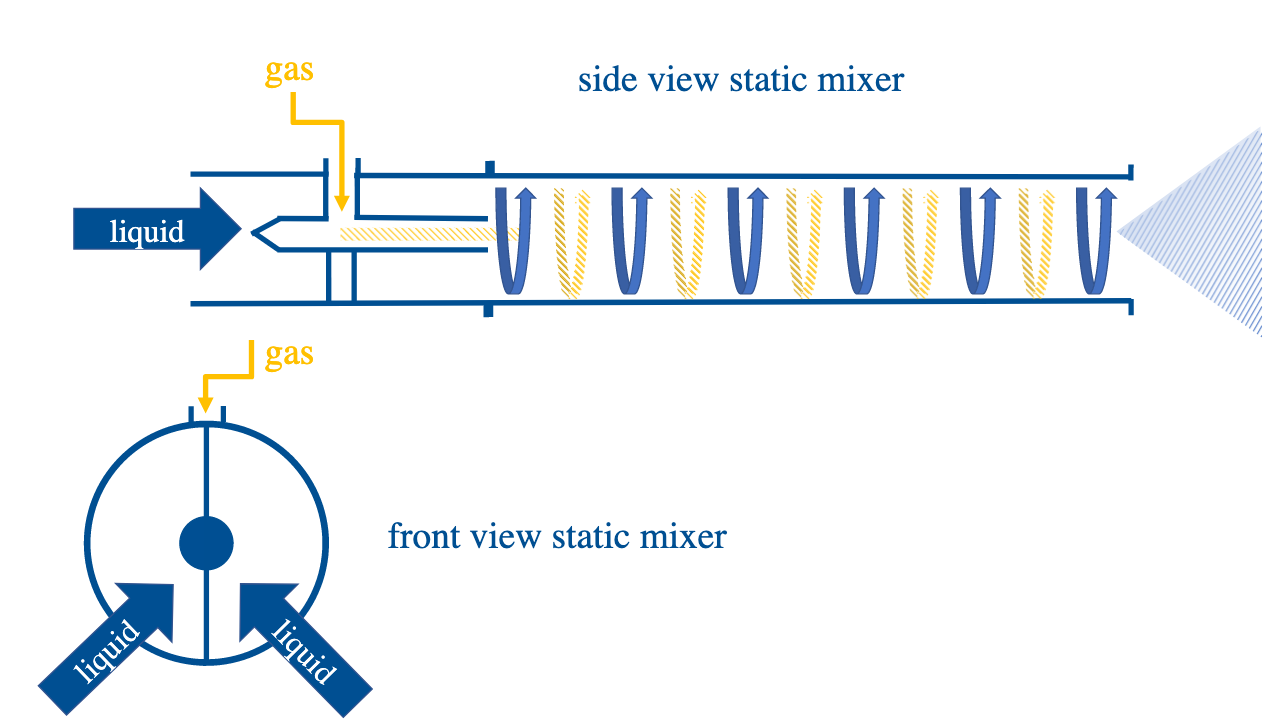
A turbulent static mixer mixes a liquid and a gas by creating a vortex. The inflow of gas breaks the liquid vortex and nanobubbles are formed where water and gas collide. This technology is less energy intensive than the previous two.
There are various other systems for nanobubble generation, for example, ejector nozzle and hammermill rotation.
Applications
Nanobubble generators are modular and can scale up easily. Pumped capacity ranges from 50 L/minute to 4 000 L/minute. Manufacturers generally offer rental leases and have reported reaching return on investment within 14 months.
Nanobubble systems function well within closed production systems (e.g. hatcheries) and semi-closed production systems (e.g. skirted salmon cages), especially for salmonid species with high stocking densities and low tolerance to low DO. Researchers also discovered that nanobubble aeration in biofloc pond systems for shrimp and tilapia work especially well to keep the flocs from settling and to prevent dead zones.
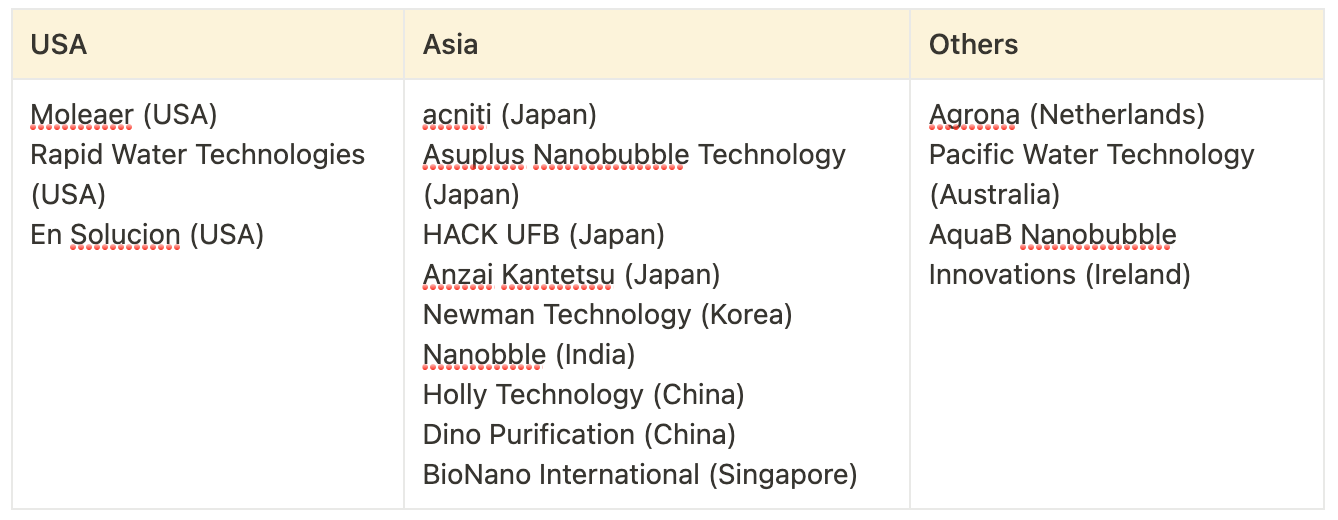
Key Nanobubble Manufacturers (by Country)
References
The Fish Site - Nanobubbles lead to major oxygen savings in salmon farms
The Fish Site - A breath of fresh air: how nanobubbles can make aquaculture more sustainable
Zeta potential - What is it and how can it be characterized?
Wiki - Zeta potential
Environmental Engineering Science - Stability of Nanobubbles
FAO - Sustainable Intensification of Aquaculture Using Efficient Nanobubble Technology




This is a great summary of the technology, thanks for the post!